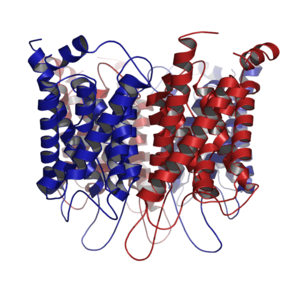
Aquaporins, also called water channels, are integral membrane proteins from a larger family of major intrinsic proteins that form pores in the membrane of biological cells, mainly facilitating transport of water between cells.[1] The cell membranes of a variety of different bacteria, fungi, animal and plant cells contain aquaporins through which water can flow more rapidly into and out of the cell than by diffusing through the phospholipid bilayer.[2] Aquaporin has six membrane-spanning alpha helical domains with both carboxylic and amino terminals on the cytoplasmic side. Two hydrophobic loops contain conserved asparagine-proline-alanine NPA motif.[further explanation needed] Because aquaporin is usually always open and is prevalent in just about every cell type, this leads to a misconception that water readily passes through the cell membrane down its concentration gradient. This is not true because only non-polar substances can diffuse directly through the lipid bilayer.